Abstract
BACKGROUND:
Initial response to standard induction therapy for acute myeloid leukemia (AML) is typically assessed by a bone marrow biopsy on day 14 (D14). However, there does not appear to be a consensus on who should receive re-induction therapy and what regimen to choose if treatment is given. Fludarabine, high dose cytarabine and granulocyte colony stimulating factor (FLAG) regimen has been used at our institution for re-induction in patients with residual disease on D14 marrow evaluation.
METHODS:
A retrospective chart review was performed to evaluate adult patients with AML who received re-induction with FLAG for residual leukemia on D14 marrow between September 2012 and July 2017 at our institution. All patients included in the analysis received standard induction chemotherapy with cytarabine and an anthracycline. Approval for the study was obtained from the IRB.
RESULTS:
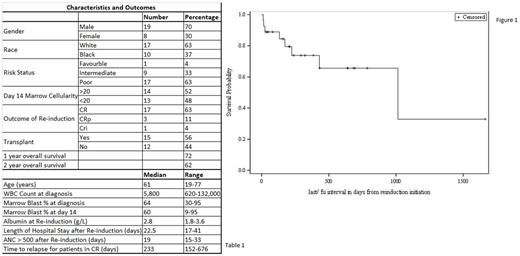
We identified 27 patients that received FLAG as re-induction (Table 1). The median age at diagnosis was 61 years (range 19-77). Median WBC count at presentation was 5.8 X10^3/ml (range 0.6-132) and bone marrow blast % was 64 (range 30-95). Most patients (63%) had poor risk disease based on cytogenetics and molecular abnormalities (as per NCCN AML guidelines), one patient (4%) had favorable risk disease with the rest of the patients (33%) having intermediate risk disease. The D14 marrow had a minimum cellularity of more than 20% in 14 patients. The median blast % by morphology on the D14 marrow was 60% (range 9-95). The overall response rate (CR/CRp/CRi) was 78% with 17 out of 27 (63%) patients achieving complete remission (CR). Four patients (15%) had resistant disease after FLAG based on a bone marrow evaluation and 2 patients died of indeterminate cause. Of the 4 patients with resistant disease, 1 achieved CR with a second cycle of FLAG. Overall, three patients died in the hospital during re-induction.
The median time period for neutrophil count recovery (ANC >500) from re-induction date was 19 days (range 15-33). The median length of hospital stay after re-induction for patients that were discharged was 22.5 days (range 17-41). Fifteen patients (56%) proceeded to hematopoietic stem cell transplant (HSCT). Out of the 15, 14 were in CR1 at the time of HSCT. At the last follow up, 16 patients were in remission. The median follow up time period after re-induction was 224 days (range 11-1,663). The median time to relapse for patients that achieved CR after initiating FLAG re-induction was 233 days (range 152-676). The 1 and 2 year overall survival (OS) rates were 72% and 62% respectively (Figure 1).
CONCLUSION:
We report favorable outcomes with FLAG as a re- induction regimen for patients found to have residual AML on D14 bone marrow biopsy following standard induction chemotherapy. The median age of our population was > 60 years and 63% of the patients had poor risk AML. Within the limitations of a small retrospective study, FLAG was well tolerated with 3 patients expiring during the initial hospitalization. For AML patients with residual disease 14 days after standard induction therapy and who are able to receive further treatment, FLAG is a reasonable salvage option.
Erba: Celgene: Consultancy, Other: Chair, Scientific Steering Committee , Speakers Bureau; Incyte: all research support paid to University of Alabama, Consultancy, Speakers Bureau; Jazz: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Amgen: Consultancy, Other: all research support paid to University of Alabama, Research Funding; Daiichi Sankyo: Consultancy, Other: all research support paid to University of Alabama, Research Funding; ImmunoGen: Consultancy, Other: all research support paid to University of Alabama, Research Funding; MacroGen: Consultancy; Ono: Consultancy; Pfizer: Consultancy; Seattle Genetics: Consultancy, Other: all research support paid to University of Alabama, Research Funding; Sunesis: Consultancy; Millennium/Takeda: Consultancy, Other: all research support paid to University of Alabama, Research Funding; Agios: Other: all research support paid to University of Alabama, Research Funding; Juno: Other: all research support paid to University of Alabama, Research Funding; Astellas: Other: all research support paid to University of Alabama, Research Funding; Celator: Other: all research support paid to University of Alabama, Research Funding; Janssen: Other: all research support paid to University of Alabama, Research Funding; Glycomimetics: Other: Chair, Data and Safety Monitoring Committee.
Author notes
Asterisk with author names denotes non-ASH members.